Calcium carbide (CaC2) reacts with water to form acetylene (C2H2) and Ca(OH)2. From the following enthalpy of reaction data and data in Appendix C, calculate H°f for CaC2(s): CaC2(s) + 2 H2O(l) → Ca(OH2)(s) + C2H2(g) ΔH° = -127.2 kJ
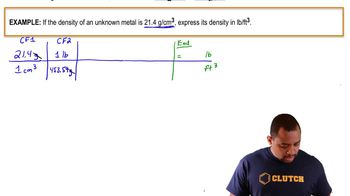
Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (c) Calculate the heat produced per liter of ethanol by combustion of ethanol under constant pressure. Ethanol has a density of 0.789 g/mL.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Combustion Reaction

Heat of Combustion

Density and Volume Conversion
Gasoline is composed primarily of hydrocarbons, including many with eight carbon atoms, called octanes. One of the cleanest–burning octanes is a compound called 2,3,4- trimethylpentane, which has the following structural formula: The complete combustion of one mole of this compound to CO2(g) and H2O(g) leads to ΔH° = -5064.9 kJ. (b) By using the information in this problem and data in Table 5.3, calculate H°f for 2,3,4-trimethylpentane.
Diethyl ether, C4H10O(l), a flammable compound that was once used as a surgical anesthetic, has the structure The complete combustion of 1 mol of C4H10O(l) to CO2(g) and H2O(l) yields ΔH° = -2723.7 kJ. (a) Write a balanced equation for the combustion of 1 mol of C4H10O(l).
Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (d) Calculate the mass of CO2 produced per kJ of heat emitted.
Methanol (CH3OH) is used as a fuel in race cars. (b) Calculate the standard enthalpy change for the reaction, assuming H2O(g) as a product.
Methanol (CH3OH) is used as a fuel in race cars. (c) Calculate the heat produced by combustion per liter of methanol. Methanol has a density of 0.791 g/mL.
