You know that an unlabeled bottle contains an aqueous solution of one of the following: AgNO3,CaCl2, or Al2(SO4)3. You take a portion of the solution and add an aqueous solution of Ba(NO3)2 to it, and observe that a white solid precipitates Then you take another portion of the unlabeled solution and add an aqueous solution of NaCl to it; nothing appears to happen. What is the most likely identity of the solution in the unlabeled bottle: silver nitrate, calcium chloride, aluminum sulfate?
Ch.4 - Reactions in Aqueous Solution
Chapter 4, Problem 32
Which of the following solutions is the most basic? a. 0.6𝑀 NH3 b. 0.150 M KOH c. 0.100𝑀Ba(OH)2
 Verified step by step guidance
Verified step by step guidance1
Identify the nature of each compound: NH3 is a weak base, KOH is a strong base, and Ba(OH)2 is a strong base.
For strong bases like KOH and Ba(OH)2, determine the concentration of hydroxide ions (OH⁻) they produce. KOH dissociates completely to give 0.150 M OH⁻, while Ba(OH)2 dissociates to give 0.200 M OH⁻ (since it produces two OH⁻ ions per formula unit).
For the weak base NH3, use the base dissociation constant (Kb) to estimate the concentration of OH⁻ ions it produces. This requires setting up an equilibrium expression and solving for OH⁻ concentration.
Compare the OH⁻ concentrations from each solution: higher OH⁻ concentration indicates a more basic solution.
Determine which solution has the highest OH⁻ concentration to identify the most basic solution.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
pH and Basicity
pH is a measure of the hydrogen ion concentration in a solution, indicating its acidity or basicity. A pH greater than 7 signifies a basic solution. Basicity refers to the ability of a substance to accept protons (H+) or donate electron pairs, with stronger bases resulting in higher pH values.
Recommended video:
Guided course

The pH Scale
Concentration of Hydroxide Ions
The basicity of a solution is often determined by the concentration of hydroxide ions (OH-). Strong bases, such as KOH and Ba(OH)2, dissociate completely in water, increasing the OH- concentration. The more hydroxide ions present, the more basic the solution will be.
Recommended video:
Guided course
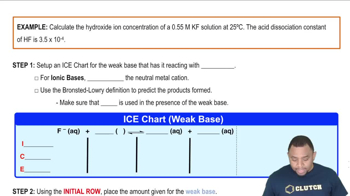
Hydroxide Ion Concentration Example
Dissociation of Bases
Different bases dissociate in water to varying extents. For example, KOH is a strong base that fully dissociates into K+ and OH-, while Ba(OH)2 also dissociates completely, producing two moles of OH- per mole of Ba(OH)2. NH3, on the other hand, is a weak base that does not fully dissociate, making its contribution to basicity less significant compared to strong bases.
Recommended video:
Guided course

Acid-Base Dissociation Example
Related Practice
Textbook Question
3
views
Textbook Question
Three solutions are mixed together to form a single solution; in the final solution, there are 0.2 mol Pb1CH3COO)2, 0.1 mol Na2S, and 0.1 mol CaCl2 present. What solid(s) will precipitate?
2338
views
4
rank
Textbook Question
Which of the following solutions is the most acidic? a. 0.2 M LiOH b. 0.2 M HI c. 1.0 M methanol (CH3OH)
Textbook Question
State whether each of the following statements is true or false. Justify your answer in each case. (a) Sulfuric acid is a monoprotic acid.
341
views
Textbook Question
State whether each of the following statements is true or false. Justify your answer in each case. (b) HCl is a weak acid.
329
views
Textbook Question
State whether each of the following statements is true or false. Justify your answer in each case. (c) Methanol is a base.
360
views
