Here are the essential concepts you must grasp in order to answer the question correctly.
Beta Decay
Beta decay is a type of radioactive decay in which a nucleus emits a beta particle, which can be an electron (beta-minus decay) or a positron (beta-plus decay). In beta-minus decay, a neutron is converted into a proton, increasing the atomic number by one, while in beta-plus decay, a proton is converted into a neutron, decreasing the atomic number by one. This process helps unstable nuclei achieve a more stable configuration.
Recommended video:
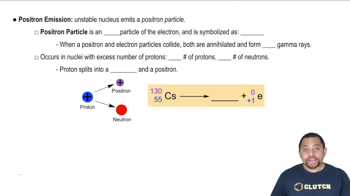
Positron Emission
Positron emission, also known as beta-plus decay, occurs when a proton in a nucleus is transformed into a neutron, resulting in the emission of a positron (the antimatter counterpart of an electron). This process decreases the atomic number of the element by one while keeping the mass number unchanged. Positron emission is common in isotopes that are proton-rich and seek stability.
Recommended video:
Tritium
Tritium is a radioactive isotope of hydrogen with one proton and two neutrons, represented as 3H. It is unstable and undergoes beta decay to achieve stability. In the case of tritium, it emits a beta particle (electron) as it decays into helium-3, which has one less neutron, thus illustrating the process of beta-minus decay.
Recommended video:
Hydrogen Isotopes Example
 Verified step by step guidance
Verified step by step guidance