You are asked to prepare a pH = 3.00 buffer solution starting from 1.25 L of a 1.00 M solution of hydrofluoric acid (HF) and any amount you need of sodium fluoride (NaF). (b) How many grams of sodium fluoride should be added to prepare the buffer solution? Neglect the small volume change that occurs when the sodium fluoride is added.

A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. (a) What is the pH of this buffer?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Buffer Solutions

Henderson-Hasselbalch Equation

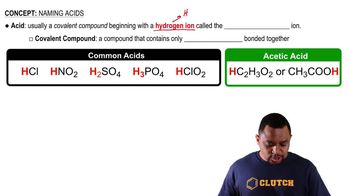
Acetic Acid and Sodium Acetate
You are asked to prepare a pH = 4.00 buffer starting from 1.50 L of 0.0200 M solution of benzoic acid 1C6H5COOH2 and any amount you need of sodium benzoate 1C6H5COONa2. (a) What is the pH of the benzoic acid solution prior to adding sodium benzoate?
You are asked to prepare a pH = 4.00 buffer starting from 1.50 L of 0.0200 M solution of benzoic acid 1C6H5COOH2 and any amount you need of sodium benzoate 1C6H5COONa2. (b) How many grams of sodium benzoate should be added to prepare the buffer? Neglect the small volume change that occurs when the sodium benzoate is added.
A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. b. What is the pH of the buffer after the addition of 0.020 mol of KOH?
A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. c. What is the pH of the buffer after the addition of 0.020 mol of HNO3?
