Brass is a substitutional alloy consisting of a solution of copper and zinc. A particular sample of red brass consisting of 80.0 % Cu and 20.0 % Zn by mass has a density of 8750 kg/m3. (a) What is the molality of Zn in the solid solution?
Suppose that you want to use reverse osmosis to reduce the salt content of brackish water containing 0.22 M total salt concentration to a value of 0.01 M, thus rendering it usable for human consumption. What is the minimum pressure that needs to be applied in the permeators (Figure 13.26) to achieve this goal, assuming that the operation occurs at 298 K?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Reverse Osmosis

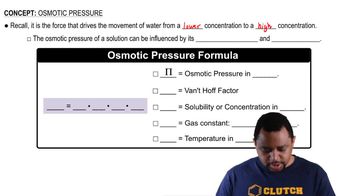
Osmotic Pressure
Ideal Gas Law and Temperature

Brass is a substitutional alloy consisting of a solution of copper and zinc. A particular sample of red brass consisting of 80.0 % Cu and 20.0 % Zn by mass has a density of 8750 kg/m3. (b) What is the molarity of Zn in the solution?
Caffeine (C8H10N4O2) is a stimulant found in coffee and tea. If a solution of caffeine in the solvent chloroform (CHCl3) has a concentration of 0.0500 m, calculate (b) the mole fraction of caffeine in the solution.
Assume that a portable reverse-osmosis apparatus operates on seawater, whose effective concentration (the concentration of dissolved ions) is 1.12 M, and that the desalinated water output has an effective molarity of about 0.02 M. What minimum pressure must be applied by hand pumping at 297 K to cause reverse osmosis to occur?
You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false.
a. The solid that forms as the solution freezes is nearly pure solute.
You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false.
b. The freezing point of the solution is independent of the concentration of the solute.
