What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (d) and metallic crystals?
Ch.12 - Solids and Modern Materials
Chapter 12, Problem 14c
Which type (or types) of crystalline solid is characterized by each of the following? (c) high melting point and poor electrical conductivity;
 Verified step by step guidance
Verified step by step guidance1
Identify the types of crystalline solids: molecular, ionic, covalent network, and metallic.
Consider the properties of each type: molecular solids have low melting points and are poor conductors; ionic solids have high melting points and conduct electricity when molten; covalent network solids have high melting points and are poor conductors; metallic solids have variable melting points and are good conductors.
Focus on the given properties: high melting point and poor electrical conductivity.
Match these properties to the type of crystalline solid: covalent network solids are known for having high melting points and being poor conductors of electricity.
Conclude that the type of crystalline solid characterized by a high melting point and poor electrical conductivity is a covalent network solid.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Types of Crystalline Solids
Crystalline solids can be categorized into several types based on the nature of the bonding and the arrangement of their constituent particles. The main types include ionic, covalent network, metallic, and molecular solids. Each type exhibits distinct physical properties, such as melting points and electrical conductivity, which are influenced by the strength and type of interactions between the particles.
Recommended video:
Guided course

Crystalline Solids Structure
Ionic Solids
Ionic solids are formed from the electrostatic attraction between positively and negatively charged ions. They typically have high melting points due to the strong ionic bonds that require significant energy to break. However, in their solid state, ionic solids do not conduct electricity, as the ions are fixed in place and cannot move freely, making them poor electrical conductors.
Recommended video:
Guided course

Ionic Solid Identification Example
Covalent Network Solids
Covalent network solids consist of atoms connected by a network of covalent bonds, forming a rigid structure. Examples include diamond and silicon carbide, which exhibit very high melting points due to the strength of the covalent bonds. Like ionic solids, covalent network solids also lack free-moving charged particles in their solid state, resulting in poor electrical conductivity.
Recommended video:
Guided course
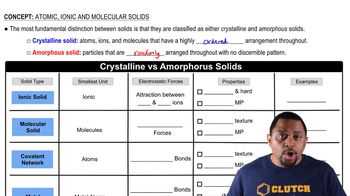
Crystalline vs Amorphous Solids
Related Practice
Textbook Question
519
views
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (a) High mobility of electrons throughout the solid;
1063
views
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (b) softness, relatively low melting point;
781
views
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.
710
views
Textbook Question
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)
1258
views
Textbook Question
You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
500
views
