In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (c) SbCl5 and AlF3.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ionic vs. Molecular Compounds

Naming Ionic Compounds

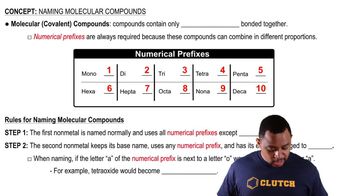
Naming Molecular Compounds
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) SiF4 and LaF3 (for ionic or molecular substances) to assign a name to each compound: (b) FeCl2 and ReCl6 (c) PbCl4 and RbCl.
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) TiCl4 and CaF2 (b) ClF3 and VF3
Draw Lewis structures for the following: (a) CH2Cl2 (b) ClCN (c) SF2
Draw Lewis structures for the following: (d) H2SO4 (H is bonded to O) (e) OF2
Draw Lewis structures for the following: (f) NH2OH
