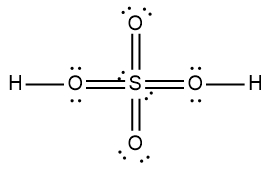
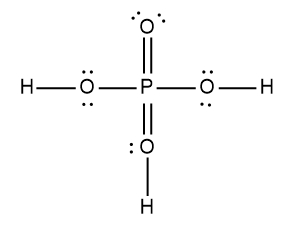
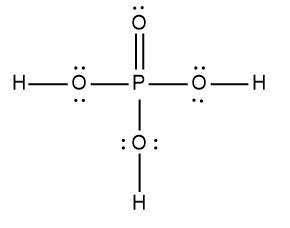
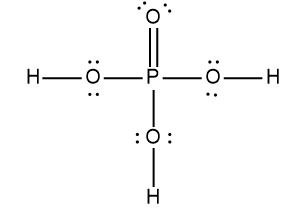
Here it says to draw the Lewis dot structure for the nitric acid molecule. So HNL3 we're going to step zero, we're going to break up the acid into the H ion and an anion. Now we're going to say here that for the anion, follow steps one to six to draw its Lewis dot structure. All right, so nitric acid is H+ and then NO3-, NO3- is our anion.
Nitrogen is in Group 5A, so it has five valence electrons. Oxygen has six because it's in Group 6A, so it's going to be 5 + 18 -. 1 means we've gained an electron from an outside source, so that's 24 valence electrons. Let's draw it. Nitrogen goes in the center, it's going to form single bonds with the oxygens. Now remember we need to follow the octet rule for the surrounding elements, so we're adding electrons from.
Now each oxygen has 8 electrons around them, but we've run into an issue with the nitrogen. It only has two 46 electrons around itself. To get it to also follow the octet rule, what do we do? Well, we're going to erase one of these lone pairs and use it to make a double bond. Because nitrate ion has a negative charge, we're going to place it in brackets and the charge on the outside. So this is the drawing of the nitrate ion.
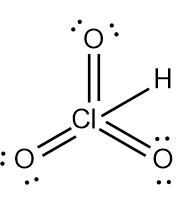
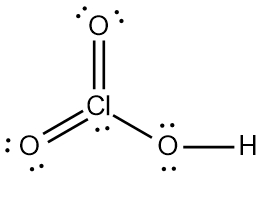
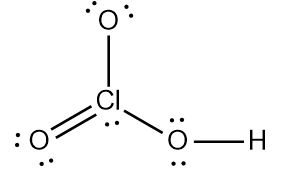
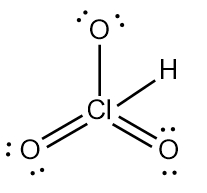
Step 7 says add the H ion to the surrounding elements with a form onto the surrounding elements with a formal charge of -1. Now here, who's gonna have a formal charge of -1? It'll be one of the oxygens that has a formal charge of -1. So here remember formal charge equals group number minus the bonds the element is making plus each individual non bonding electron. So if we looked at this oxygen here, it's 6 -, 1 bond plus six, which equals -1. So what I'm going to do here is I'm going to erase one of these lone pairs here and use it to connect to the hydrogen. So here this here represents the nitric acid molecule and would be the correct representation for this particular.