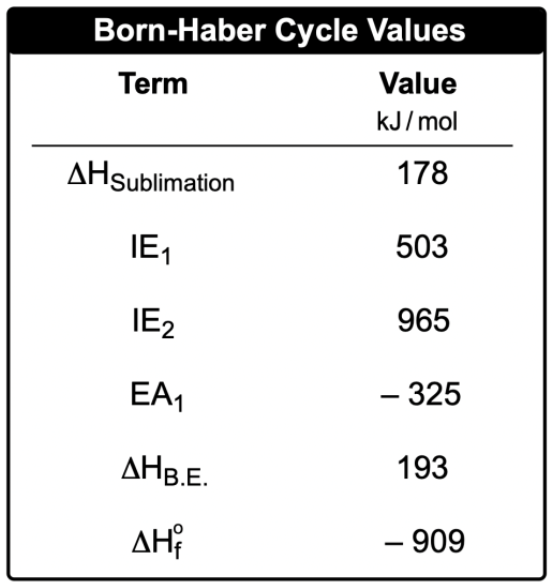
Now the Borne Haber cycle is a reaction outline that gives the steps for the formation of an ionic compound from the standard states of its elements. So I know that's a mouthful. The Born Heber cycle is an extensive process. We're going to go piece by piece, step by step to figure it out.
Now recall first a formation equation shows the standard states of elements combining to form 1 mole of product. Now remember, associated with the formation equation is our enthalpy or heat of formation which is ΔHf. Here standard heat of formation has that little circle there. It's telling me here that the formation of 1 mole of sodium chloride is accomplished by combining 1 mole of sodium solid with half a mole of chlorine gas. Remember in formation equations we can have fractions and decimals as coefficients.
Now in order to calculate the enthalpy of formation, both elements must first be converted into their ionic gaseous forms. So we're starting out here with sodium solid. We're starting out here with Cl2 gas. What we need to do first is realize that we need to get sodium to its ionic form as a gas, and we need to get chlorine to its ionic form as a gas as well. To do that, I first have to convert sodium solid into a gas. Going from a solid to a gas is called sublimation. So we're dealing with enthalpy of sublimation.
Once I have it in its gaseous state, I need to change it from a neutral metal to a positively charged metal. That means I'm removing electrons. Removing electrons has to do with ionization energy, and since I'm only removing one electron, its ionization energy one. If I needed to remove more than one electron, then I'd have to add an ionization 2, ionization 3, and so on. Remember we talked about this under successive ionization energy's.
Next Here I need to separate Cl2 into two separate chlorines, so I have to break the bond. This is called enthalpy of dissociation. Here, once we break the bond between the two chlorines, I just have one chlorine now as a gas, and then I have to add an electron to it. Adding an electron is an electron affinity, and since I'm only adding one electron, it's EA one. If I had to add more than one electron, there would be EA2EA3 and so on.
Now that I have both of them in their ionic forms, they're going to combine together to give me my solid. Remember combining gaseous ions to form a solid ionic product? This is connected to lattice energy, so it's a whole process. We have to do all of this just to get the enthalpy of formation. So I need to do step one which is enthalpy of sublimation. Step 2 would be ionization energy which could be in more than one step. Step three I have to do enthalpy of dissociation to break a bond so I can get a single chlorine. Then I need to make it negative. So what I have to do? Electron affinity, which also could be more than one step. Once I get them into their ionic gas form, then I have lattice energy to complete it.
O. You can say that enthalpy of formation is composed of these five fundamental steps. So like I told you, the Born Haber cycle has a lot of parts to it. But just remember the method we used here to justify how sodium combines with chlorine to give us sodium chloride.