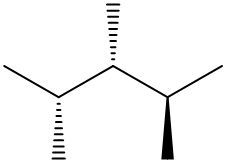
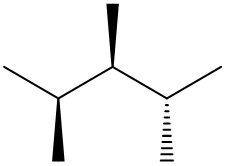
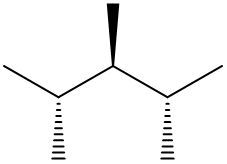
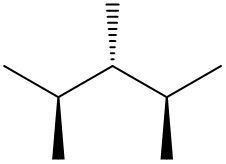
In this video, we're going to take a look at spatial orientation of bonds. Now we're going to say that the skeletal formula also shows how atoms in the molecule are arranged in space in addition to how atoms are bonded. Here we're going to say that atoms or groups on a solid wedge come out of the page, meaning they're above the plane towards the observer. So imagine you're looking at this molecule that OH is pointing straight up at you towards your face. So here this is our solid wedge, meaning that OH is pointing straight out of the paper towards you.
We're going to say next that atoms on dashed wedges go inside the page, they lie below the plane and we're going to say they are away from the observer. So here's our dashed wedged line. That means it goes into the page. So if you want to think about this in terms of 3D, imagine my hand here is a piece of paper. We'd say that the OH is a solid wedge, so it's pointing up towards my face, towards my chin. And then we'd say that this group here, which is a chapter three group, is dash. So it's below. It's on the bottom away from my chin.
So that's what we mean in terms of orientation in space. We can actually say what direction the group is pointing now based on if it's a solid wedge or a dashed wedge. So hopefully that makes more sense. As we delve deeper into skeletal formulas, we'll see these types of bonds pop up here and there.