Writing Ionic Compounds definitions Flashcards
 Back
BackWriting Ionic Compounds definitions
1/15
Terms in this set (15)
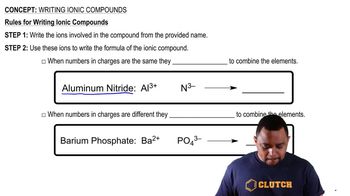
- Ionic CompoundsCompounds formed by the electrostatic attraction between positive and negative ions.
- AluminumA metal in group 3A of the periodic table with a common charge of +3.
- NitrideA non-metal ion derived from nitrogen, typically carrying a -3 charge.
- BariumAn alkaline earth metal in group 2A with a common charge of +2.
- PhosphateA polyatomic ion with the formula PO4 and a charge of -3.
- Crisscross MethodA technique to balance ionic charges by swapping the magnitude of charges between ions.
- Polyatomic IonAn ion composed of two or more atoms covalently bonded, acting as a single charged entity.
- Periodic TableA tabular arrangement of elements by increasing atomic number, showing periodic trends.
- Group 3AA column in the periodic table where elements typically have a +3 charge.
- Group 2AA column in the periodic table where elements typically have a +2 charge.
- ChargeAn electrical property of ions, determining their attraction or repulsion in compounds.
- FormulaA representation of the elements in a compound and their ratios.
- Non-metalElements typically found on the right side of the periodic table, often forming anions.
- TetraoxidesCompounds containing four oxygen atoms, often part of polyatomic ions.
- ParenthesisUsed in chemical formulas to indicate the number of polyatomic ions in a compound.