Valence Electrons of Elements definitions Flashcards
 Back
BackValence Electrons of Elements definitions
1/15
Terms in this set (15)
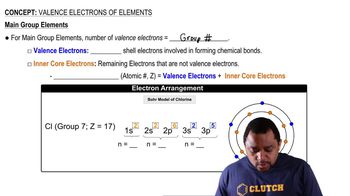
- Valence ElectronsOuter shell electrons involved in forming chemical bonds.
- Main Group ElementsElements whose valence electrons equal their group number.
- Transition MetalsGroup B elements with valence electrons from s and d orbitals.
- Electron ConfigurationArrangement of electrons in an atom's orbitals.
- Atomic NumberTotal number of protons in an atom, equal to total electrons.
- Inner Core ElectronsElectrons not involved in bonding, located in inner shells.
- Group NumberNumber indicating an element's column in the periodic table.
- s OrbitalSpherical orbital that can hold up to two electrons.
- d OrbitalOrbital with complex shapes, can hold up to ten electrons.
- Periodic TableChart organizing elements by increasing atomic number.
- Chemical BondsAttractions between atoms that hold compounds together.
- Group 7AColumn in the periodic table with seven valence electrons.
- Group 3 to 12Transition metal groups with valence electrons equal to group number.
- Shell NumberEnergy level of an electron in an atom, indicated by a number.
- ChlorineElement in group 7A with seven valence electrons.