Triprotic Acids and Bases Calculations definitions Flashcards
 Back
BackTriprotic Acids and Bases Calculations definitions
1/10
Terms in this set (10)
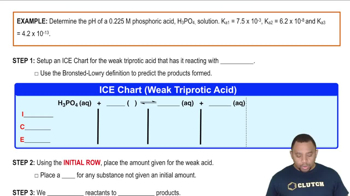
- Triprotic AcidsAcids capable of donating three protons, existing in four forms: fully protonated, two intermediates, and deprotonated.
- Acidic FormThe form of a triprotic acid where all three protons are present, used in pH calculations with Ka1.
- Basic FormThe deprotonated form of a triprotic acid, used in pH calculations with Kb1.
- Intermediate FormForms of a triprotic acid where one or two protons have been lost, not primarily used in pH calculations.
- Ka1The acid ionization constant used to calculate the pH of the fully protonated form of a triprotic acid.
- Kb1The base ionization constant used to calculate the pH of the deprotonated form of a triprotic acid.
- IonizationThe process by which an acid loses protons, significant in determining the pH of triprotic acids.
- ProtonatedA state where an acid retains its protons, crucial for understanding the acidic form in triprotic acids.
- DeprotonatedA state where an acid has lost its protons, relevant for the basic form in triprotic acids.
- pH CalculationThe process of determining the acidity or basicity of a solution, focusing on the initial forms of triprotic acids.