Titrations: Strong Acid-Strong Base definitions Flashcards
 Back
BackTitrations: Strong Acid-Strong Base definitions
1/12
Terms in this set (12)
- TitrationA process in chemistry where a solution of known concentration is used to determine the concentration of an unknown solution.
- Strong AcidA type of acid that completely dissociates in solution, providing a high concentration of hydrogen ions.
- Strong BaseA type of base that completely dissociates in solution, providing a high concentration of hydroxide ions.
- TitrantThe solution of known concentration added to another solution to determine its concentration during titration.
- TitrateThe solution of unknown concentration in a titration process that reacts with the titrant.
- NeutralizationA chemical reaction between an acid and a base that results in the formation of water and a salt.
- pHA scale used to specify the acidity or basicity of an aqueous solution, crucial in titration calculations.
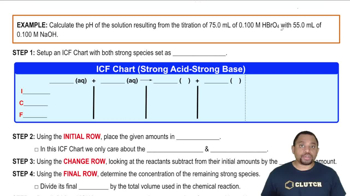
- ICE ChartA tool used to track the initial, change, and final concentrations of reactants and products in a chemical reaction.
- ICF ChartA variation of the ICE chart used specifically in titrations to determine pH changes.
- ConcentrationThe amount of a substance per defined space, crucial in determining the strength of acids and bases.
- ReactionA process in which substances interact to form new substances, fundamental in titration.
- SaltAn ionic compound formed from the neutralization reaction of an acid and a base.