Third Law of Thermodynamics definitions Flashcards
 Back
BackThird Law of Thermodynamics definitions
1/10
Terms in this set (10)
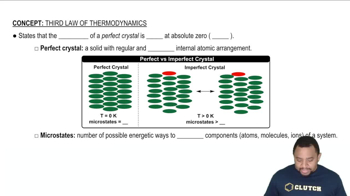
- Third Law of ThermodynamicsStates that the entropy of a perfect crystal is zero at absolute zero temperature.
- EntropyA measure of disorder in a system, linked to the number of possible microstates.
- Perfect CrystalA solid with a regular and ideal internal atomic arrangement at 0 Kelvin.
- Absolute ZeroThe lowest possible temperature, 0 Kelvin, where atomic motion ceases.
- MicrostateThe number of possible energetic arrangements of a system's components.
- Boltzmann EquationRelates entropy to microstates using the formula S = k ln(W).
- Boltzmann ConstantA constant (1.38 x 10^-23 J/K) used in the Boltzmann equation to calculate entropy.
- KelvinThe SI unit of temperature, where 0 Kelvin is absolute zero.
- Ludwig BoltzmannAustrian physicist who formulated the equation relating entropy to microstates.
- DisorderThe degree of randomness or chaos in a system, associated with higher entropy.