The Electron Configuration Review definitions Flashcards
 Back
BackThe Electron Configuration Review definitions
1/15
Terms in this set (15)
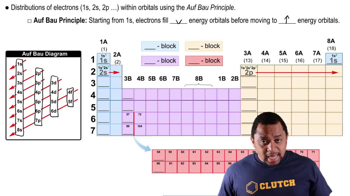
- Electron ConfigurationRepresents the distribution of electrons in an atom's orbitals, following a specific sequence.
- Aufbau PrincipleElectrons fill lower energy orbitals before occupying higher energy ones.
- OrbitalA region in an atom where there is a high probability of finding electrons.
- Periodic TableA tabular arrangement of elements, organized by increasing atomic number and electron configuration.
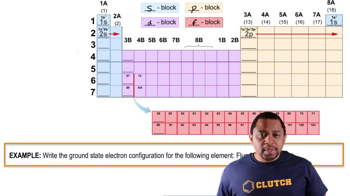
- S BlockPart of the periodic table where elements have their outermost electrons in s orbitals.
- P BlockPart of the periodic table where elements have their outermost electrons in p orbitals.
- D BlockPart of the periodic table where elements have their outermost electrons in d orbitals.
- F BlockPart of the periodic table where elements have their outermost electrons in f orbitals.
- Condensed Electron ConfigurationA shorthand notation using the last noble gas to simplify electron arrangements.
- Noble GasElements in group 18 of the periodic table, used as reference points in electron configurations.
- Ground StateThe lowest energy state of an atom, with electrons in the lowest possible orbitals.
- SublevelA division of electron shells in an atom, consisting of orbitals of the same energy level.
- Energy LevelThe fixed amount of energy that a system described by quantum mechanics can have.
- ElectronA subatomic particle with a negative charge, found in orbitals around an atom's nucleus.
- IonAn atom or molecule with a net electric charge due to the loss or gain of one or more electrons.