The Electron Configuration: Quantum Numbers definitions Flashcards
 Back
BackThe Electron Configuration: Quantum Numbers definitions
1/15
Terms in this set (15)
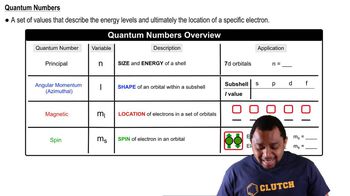
- Quantum NumbersA set of values describing the energy levels and location of electrons in an atom.
- Principal Quantum NumberIndicates the size and energy of an electron shell, represented by the variable n.
- Angular Momentum Quantum NumberDefines the shape of orbitals within a subshell, represented by the variable l.
- Magnetic Quantum NumberSpecifies the orientation of an orbital within a subshell, represented by the variable ml.
- Spin Quantum NumberIndicates the spin of an electron within an orbital, represented by the variable ms.
- Aufbau PrincipleDictates the order of electron filling in orbitals, starting from the lowest energy level.
- Electron ConfigurationThe arrangement of electrons in an atom's orbitals, following specific rules and principles.
- OrbitalA region in an atom where there is a high probability of finding electrons.
- SubshellA division of electron shells, characterized by specific shapes and energy levels.
- s OrbitalA spherical orbital with an angular momentum quantum number of 0.
- p OrbitalA dumbbell-shaped orbital with an angular momentum quantum number of 1.
- d OrbitalA cloverleaf-shaped orbital with an angular momentum quantum number of 2.
- f OrbitalA complex-shaped orbital with an angular momentum quantum number of 3.
- Electron ShellA group of atomic orbitals with the same principal quantum number.
- Energy LevelThe fixed amount of energy that a system described by quantum mechanics can have.