Strong-Field vs Weak-Field Ligands definitions Flashcards
 Back
BackStrong-Field vs Weak-Field Ligands definitions
1/15
Terms in this set (15)
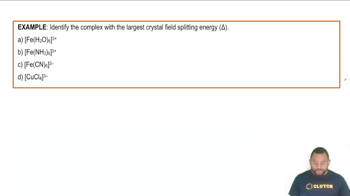
- Crystal Field Splitting EnergyEnergy difference between lower and higher orbitals in a metal complex influenced by ligand type.
- Octahedral ComplexA coordination compound where a central metal atom is surrounded by six ligands.
- Strong-Field LigandLigands that cause a large energy gap between orbitals, leading to a large crystal field splitting energy.
- Weak-Field LigandLigands that result in a small energy gap between orbitals, causing a small crystal field splitting energy.
- Degenerate OrbitalsOrbitals that have the same energy level, often seen with weak-field ligands.
- CyanideA strong-field ligand known for creating the largest crystal field splitting energy.
- IodineA weak-field ligand associated with the smallest crystal field splitting energy.
- EthylenediamineA strong-field ligand that contributes to a large crystal field splitting energy.
- AmmoniaA strong-field ligand that increases the crystal field splitting energy.
- HalogensElements in group 7A, often weak-field ligands, ordered from fluorine to iodine.
- FluorineA halogen and weak-field ligand, part of the order from strong to weak ligands.
- ChlorineA halogen and weak-field ligand, following fluorine in ligand strength.
- BromineA halogen and weak-field ligand, positioned before iodine in ligand strength.
- WaterA weak-field ligand that precedes halogens in the order of ligand strength.
- NitrateA strong-field ligand contributing to a large crystal field splitting energy.