Standard Reduction Potentials definitions Flashcards
 Back
BackStandard Reduction Potentials definitions
1/12
Terms in this set (12)
- OxidationA chemical process involving the loss of electrons by a species.
- ReductionA chemical process involving the gain of electrons by a species.
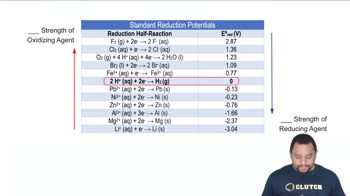
- Standard Reduction PotentialA measure of the tendency of a chemical species to gain electrons under standard conditions.
- Standard ConditionsConditions set at 25°C, 1 atmosphere pressure, 1 M concentration, and pH 7.
- Standard Hydrogen ElectrodeA reference electrode with a standard reduction potential of zero.
- Oxidizing AgentA substance that gains electrons and is reduced in a chemical reaction.
- Reducing AgentA substance that loses electrons and is oxidized in a chemical reaction.
- Half ReactionA representation of either the oxidation or reduction process in a redox reaction.
- FluorineAn element with the highest standard reduction potential, indicating strong oxidizing ability.
- LithiumAn element with a low standard reduction potential, indicating strong reducing ability.
- ElectronsSubatomic particles involved in oxidation and reduction processes.
- pHA scale used to specify the acidity or basicity of an aqueous solution.