Resonance Structures definitions Flashcards
 Back
BackResonance Structures definitions
1/10
Terms in this set (10)
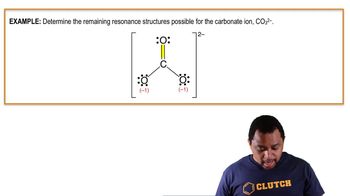
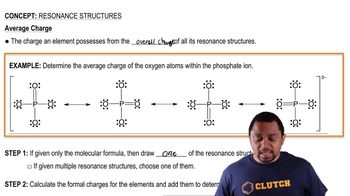
- Resonance StructuresMultiple valid Lewis structures for ions with pi bonds, showing different electron arrangements.
- Lewis Dot StructuresDiagrams showing the bonding between atoms and the lone pairs of electrons in a molecule.
- Polyatomic IonsIons composed of two or more atoms covalently bonded, acting as a single charged entity.
- Pi BondA type of covalent bond formed by the sideways overlap of atomic orbitals, allowing electron delocalization.
- Lone PairA pair of valence electrons not shared with another atom, often influencing molecular shape and reactivity.
- Nitride IonAn ion with a nitrogen atom bonded to other atoms, often depicted with resonance structures.
- Double-Sided ArrowsSymbols used to indicate the equivalency of resonance structures in depicting a molecule's structure.
- Resonance HybridThe true representation of a molecule, averaging all significant resonance structures.
- Dotted LineUsed in resonance hybrids to indicate potential locations of pi bonds across different structures.
- Electron DelocalizationThe distribution of electrons across multiple atoms, contributing to molecular stability and behavior.