Quantum Numbers: Spin Quantum Number definitions Flashcards
 Back
BackQuantum Numbers: Spin Quantum Number definitions
1/10
Terms in this set (10)
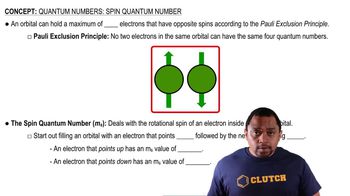
- Spin Quantum NumberDescribes the intrinsic angular momentum of electrons, denoted as ms, with values of +1/2 or -1/2.
- Pauli Exclusion PrincipleStates that no two electrons in the same orbital can have identical sets of quantum numbers.
- OrbitalA region in an atom where a maximum of two electrons with opposite spins can exist.
- Quantum NumbersSet of numbers that describe the unique quantum state of an electron in an atom.
- Intrinsic Angular MomentumThe inherent spin characteristic of an electron, contributing to its quantum state.
- Electron SpinThe rotation of an electron around its axis, influencing its ms value.
- Atomic OrbitalA space around the nucleus where electrons are likely to be found, each with a unique quantum state.
- Opposite SpinsCondition where two electrons in the same orbital have spins of +1/2 and -1/2.
- Quantum StateThe specific set of quantum numbers that describe an electron's position and energy.
- Chemical BehaviorThe reactivity and interaction of elements, influenced by electron arrangement.