Quantum Numbers: Number of Electrons definitions Flashcards
 Back
BackQuantum Numbers: Number of Electrons definitions
1/10
Terms in this set (10)
- Electron ShellA region around an atom's nucleus where electrons are likely to be found, with a capacity determined by 2n².
- Quantum NumberA value used to describe the energy levels and orbitals of electrons in an atom.
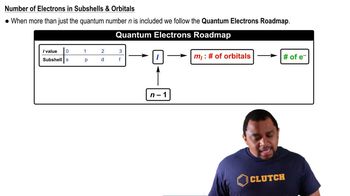
- Principal Quantum NumberDenoted as 'n', it indicates the main energy level occupied by an electron.
- OrbitalA region within an electron shell where there is a high probability of finding an electron.
- SublevelA division of electron shells based on the quantum number 'l', indicating the shape of orbitals.
- Maximum ElectronsThe highest number of electrons that can occupy a shell or orbital, calculated using 2n² for shells.
- l ValueThe azimuthal quantum number indicating the shape and type of an orbital.
- m sub lThe magnetic quantum number representing the orientation of an orbital in space.
- Electron ConfigurationThe distribution of electrons in an atom's orbitals, determined by quantum numbers.
- n minus 1A calculation used to determine all possible 'l' values for a given principal quantum number.