Quantum Numbers: Magnetic Quantum Number definitions Flashcards
 Back
BackQuantum Numbers: Magnetic Quantum Number definitions
1/12
Terms in this set (12)
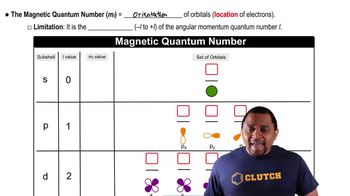
- Magnetic Quantum NumberIndicates the orientation of an electron's orbital within a subshell, ranging from -l to +l.
- OrbitalA region within a subshell where up to two electrons can be found, defined by quantum numbers.
- SubshellA division of electron shells, characterized by the angular momentum quantum number, l.
- Angular Momentum Quantum NumberDenoted as l, it determines the shape and energy level of an orbital.
- Electron ConfigurationThe distribution of electrons in an atom's orbitals, crucial for understanding chemical properties.
- s SubshellA subshell with one spherical orbital, capable of holding a maximum of 2 electrons.
- p SubshellA subshell with three orbitals, each capable of holding 2 electrons, totaling 6 electrons.
- d SubshellA subshell with five orbitals, each capable of holding 2 electrons, totaling 10 electrons.
- f SubshellA subshell with seven orbitals, each capable of holding 2 electrons, totaling 14 electrons.
- Spin Quantum NumberA quantum number that describes the intrinsic angular momentum of an electron.
- OrientationThe spatial direction of an orbital, determined by the magnetic quantum number.
- Numerical LocationThe specific position of an orbital within a subshell, indicated by ml values.