Production of Hydrogen definitions Flashcards
 Back
BackProduction of Hydrogen definitions
1/15
Terms in this set (15)
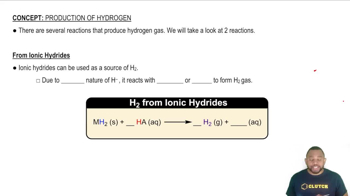
- Hydrogen gasA diatomic molecule produced from reactions involving ionic hydrides and acids or reactive metals and strong acids.
- Ionic hydridesCompounds containing hydride ions that react with acids to produce hydrogen gas and a salt.
- Hydride ionA negatively charged ion that reacts with hydrogen ions to form hydrogen gas.
- Double displacement reactionA chemical reaction where ions exchange partners, forming new compounds.
- SaltAn ionic compound formed from the reaction of an acid with a metal or hydride.
- Metal hydrideA compound of a metal with hydrogen, used to produce hydrogen gas in reactions with acids.
- Reactive metalsMetals that readily react with strong acids to produce hydrogen gas.
- Sulfuric acidA strong acid that reacts with metals to produce hydrogen gas.
- Hydrochloric acidA strong acid that reacts with metals to produce hydrogen gas.
- OxidationA process where a metal loses electrons, often producing hydrogen gas in reactions with acids.
- Group 1A metalsAlkali metals that ionize to a +1 charge when reacting with acids to produce hydrogen gas.
- Balancing equationsAdjusting coefficients in a chemical equation to ensure equal numbers of each atom on both sides.
- CoefficientA number placed before a chemical formula to balance a chemical equation.
- AnionA negatively charged ion that forms part of a salt in reactions producing hydrogen gas.
- IonizationThe process by which an atom or molecule acquires a charge by gaining or losing electrons.