Physical & Chemical Changes definitions Flashcards
 Back
BackPhysical & Chemical Changes definitions
1/15
Terms in this set (15)
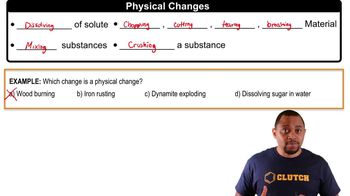
- Physical ChangeAn alteration in the physical state of a substance without changing its chemical composition.
- Chemical ChangeA transformation that alters the chemical composition, forming new substances with different identities.
- SoluteA substance that is dissolved in a liquid to form a solution.
- RustingA chemical change where metal reacts with oxygen to form a metal oxide.
- MetabolismA chemical process in living organisms that converts food into energy and new materials.
- Phase ChangeA reversible transition between different states of matter, such as solid, liquid, and gas.
- CondensationThe process where gas transforms into a liquid, often observed as water vapor on surfaces.
- DepositionA phase change where a gas turns directly into a solid without passing through the liquid state.
- FusionAnother term for melting, where a solid becomes a liquid.
- VaporizationThe process of a liquid changing into a gas, also known as evaporation.
- SublimationA phase change where a solid turns directly into a gas without becoming a liquid.
- Reversible ChangeA change that can be undone to restore the original substance, often involving phase changes.
- Irreversible ChangeA permanent transformation that cannot be undone to restore the original material.
- Chemical ReactionA process where substances undergo chemical changes to form new chemical bonds.
- Color ChangeAn indicator of a chemical change, where a substance changes color due to a reaction.