Phase Diagrams definitions Flashcards
 Back
BackPhase Diagrams definitions
1/15
Terms in this set (15)
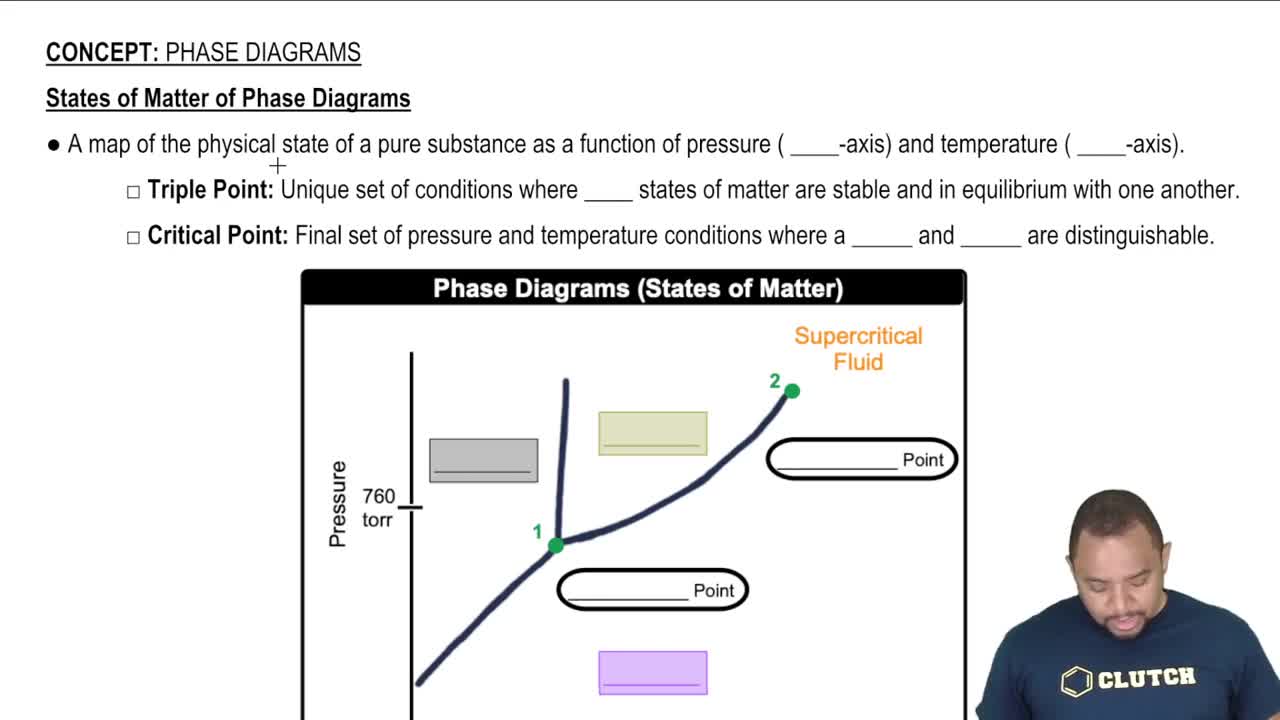
- Phase DiagramGraphical representation of states of matter of a substance under varying temperature and pressure.
- Triple PointUnique conditions where solid, liquid, and gas phases coexist in equilibrium.
- Critical PointEnd of the liquid-gas boundary where a supercritical fluid exists.
- Supercritical FluidState beyond the critical point where liquid and gas phases are indistinguishable.
- Phase ChangeReversible transition between solid, liquid, and gas states.
- Fusion CurveLine separating solid and liquid phases on a phase diagram.
- Sublimation CurveLine separating solid and gas phases on a phase diagram.
- Vaporization CurveLine separating liquid and gas phases on a phase diagram.
- Normal PressureStandard pressure of 1 atmosphere or 760 mmHg.
- Normal Melting PointTemperature where a solid becomes a liquid at normal pressure.
- Normal Boiling PointTemperature where a liquid becomes a gas at normal pressure.
- Solid PhaseState of matter with definite shape and volume, stable at low temperatures and high pressures.
- Liquid PhaseState of matter with definite volume but no fixed shape, stable at moderate temperatures and pressures.
- Gaseous PhaseState of matter with no fixed shape or volume, stable at high temperatures and low pressures.
- Phase Change CurveLine segment in a phase diagram separating two states of matter.