pH of Weak Acids definitions Flashcards
 Back
BackpH of Weak Acids definitions
1/10
Terms in this set (10)
- Weak AcidA type of acid that partially ionizes in aqueous solutions, acting as a weak electrolyte.
- Weak ElectrolyteA substance that partially dissociates into ions in solution, resulting in low electrical conductivity.
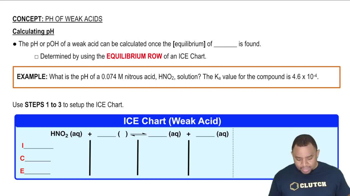
- ICE ChartA tool used to calculate equilibrium concentrations, standing for Initial, Change, Equilibrium.
- MolarityA unit of concentration measuring the number of moles of solute per liter of solution.
- Acid Dissociation ConstantA value, denoted as Ka, that quantifies the strength of a weak acid in solution.
- Percent IonizationThe percentage of a weak acid that ionizes in solution, calculated using equilibrium and initial concentrations.
- Hydronium IonA positively charged ion (H3O+) formed when an acid donates a proton to water.
- Equilibrium ConcentrationThe concentration of reactants and products in a reaction mixture when the reaction has reached equilibrium.
- Aqueous SolutionA solution in which water is the solvent.
- Strong AcidAn acid that completely ionizes in solution, acting as a strong electrolyte.