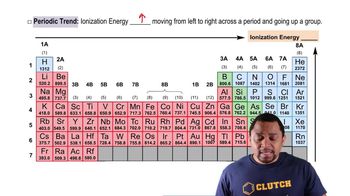
Periodic Trend: Ionization Energy definitions Flashcards
 Back
BackPeriodic Trend: Ionization Energy definitions
1/15
Terms in this set (15)
- Ionization EnergyEnergy required to remove an electron from a gaseous atom or ion, resulting in a positive charge.
- EndothermicA process where energy is absorbed, such as removing an electron from an atom.
- Potential EnergyEnergy associated with the position of an electron in an atom, related to ionization energy.
- Periodic TrendA pattern observed in the periodic table, such as the increase of ionization energy across a period.
- HeliumElement with the highest ionization energy, located at the top right of the periodic table.
- FranciumElement with the lowest ionization energy, located at the bottom left of the periodic table.
- p OrbitalsSubshells that are most stable when half-filled or fully filled, affecting ionization energy.
- s OrbitalsSubshells that are most stable when fully filled, influencing ionization energy trends.
- NitrogenElement with higher ionization energy than oxygen due to stable half-filled p orbitals.
- OxygenElement with lower ionization energy than nitrogen, willing to lose an electron for stability.
- BerylliumElement with higher ionization energy than boron due to fully filled s orbitals.
- BoronElement with lower ionization energy than beryllium, benefits from losing an electron.
- Group 6AElements with lower ionization energy than Group 5A due to electron configuration stability.
- Group 3AElements with lower ionization energy than Group 2A, influenced by s orbital stability.
- Electron ConfigurationArrangement of electrons in an atom, crucial for understanding ionization energy trends.