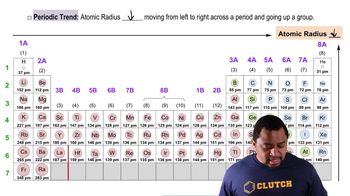
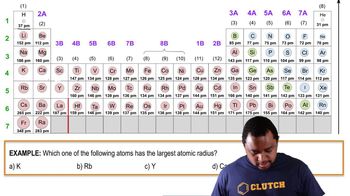
Periodic Trend: Atomic Radius definitions Flashcards
 Back
BackPeriodic Trend: Atomic Radius definitions
1/15
Terms in this set (15)
- Atomic RadiusThe distance from an atom's nucleus to its outermost electron shell.
- NucleusThe central part of an atom containing protons and neutrons.
- ProtonsPositively charged particles found in an atom's nucleus.
- NeutronsNeutral particles found in an atom's nucleus.
- Valence ShellThe outermost electron shell of an atom.
- Electron ShellsLayers around an atom's nucleus where electrons are found.
- Periodic TableA tabular arrangement of elements ordered by atomic number.
- Transition MetalsElements in the d-block of the periodic table, often with variable oxidation states.
- Inner Transition MetalsElements in the f-block, including lanthanides and actinides.
- PicometersA unit of length equal to one trillionth of a meter, used to measure atomic radii.
- Periodic TrendA pattern observed in the periodic table, such as changes in atomic radius.
- Electron AttractionThe force drawing electrons towards the nucleus, affecting atomic size.
- GroupA column in the periodic table, where elements have similar properties.
- PeriodA row in the periodic table, where elements have increasing atomic numbers.
- Synthesized ElementsElements created in laboratories, often unstable with undefined properties.