Periodic Table: Element Symbols definitions Flashcards
 Back
BackPeriodic Table: Element Symbols definitions
1/15
Terms in this set (15)
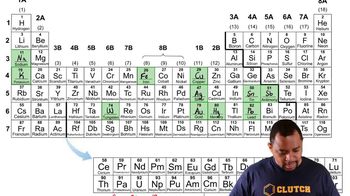
- Periodic TableA grid of elemental symbols arranged by increasing atomic number, reflecting shared chemical properties.
- ElementA substance consisting of atoms with the same number of protons, represented by a unique symbol.
- SymbolAn abbreviated form of an element's name, often derived from its English or Latin name.
- Atomic NumberThe number of protons in an element's nucleus, denoted by the variable Z.
- ProtonA positively charged particle in an atom's nucleus, determining the atomic number.
- GroupA column in the periodic table where elements share similar chemical properties.
- MendeleevA scientist who organized elements by periodic properties, predicting undiscovered elements.
- LavoisierA chemist who compiled a list of 23 known elements and their symbols in the 18th century.
- Latin NameThe original name of an element from which some symbols are derived, differing from modern names.
- SodiumAn element with the symbol Na, derived from its Latin name 'Natrium'.
- PotassiumAn element with the symbol K, derived from its Latin name 'Kalium'.
- IronAn element with the symbol Fe, derived from its Latin name 'Ferrum'.
- TungstenAn element with the symbol W, derived from its German name 'Wolfram'.
- AntimonyAn element with the symbol Sb, derived from its Latin name 'Stibium'.
- Periodic LawThe principle that elements are organized by recurring chemical properties in the periodic table.