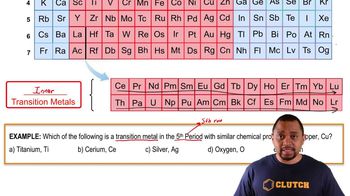
Periodic Table: Representative Elements & Transition Metals definitions Flashcards
 Back
BackPeriodic Table: Representative Elements & Transition Metals definitions
1/15
Terms in this set (15)
- Periodic TableA chart organizing elements into periods (rows) and groups (columns) based on similar properties.
- PeriodsHorizontal rows in the periodic table where elements have the same number of electron shells.
- GroupsVertical columns in the periodic table where elements share similar chemical properties.
- Transition MetalsElements in groups 3 to 12 known for variable oxidation states and multiple positive charges.
- Representative ElementsElements in groups 1, 2, and 13 to 18, also called main group or Group A elements.
- Oxidation StatesPossible charges an element can have when it forms compounds, especially in transition metals.
- Group B ElementsAnother name for transition metals, including groups 3 to 12 with unique group numbering.
- Inner Transition MetalsSubset of transition metals located between Lanthanum and Hafnium, and Actinium and Rutherfordium.
- Group A ElementsAnother term for representative elements, found in groups 1, 2, and 13 to 18.
- Main Group ElementsElements in groups 1, 2, and 13 to 18, synonymous with representative or Group A elements.
- ManganeseA transition metal with variable oxidation states, ranging from +1 to +7.
- Group 8BCollective group for elements in groups 8, 9, and 10 in the periodic table.
- Group 1BLabel for elements in group 11 of the periodic table, part of transition metals.
- Group 2BLabel for elements in group 12 of the periodic table, part of transition metals.
- LanthanumElement marking the start of the inner transition metals series in the periodic table.