Periodic Table: Group Names definitions Flashcards
 Back
BackPeriodic Table: Group Names definitions
1/15
Terms in this set (15)
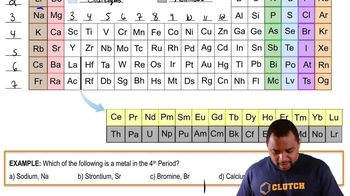
- PeriodsHorizontal rows in the periodic table, currently totaling seven, which may expand with new element discoveries.
- GroupsVertical columns in the periodic table, also known as families or series, totaling 18.
- Alkali metalsElements in Group 1, known for their high reactivity and presence in the first column.
- Alkaline earth metalsElements in Group 2, characterized by their reactivity and presence in the second column.
- PnictogensElements in Group 15, known for their varied chemical properties and presence in the nitrogen family.
- ChalcogensElements in Group 16, including oxygen, known for forming compounds with metals.
- HalogensElements in Group 17, highly reactive nonmetals, often forming salts with metals.
- Noble gasesElements in Group 18, known for their low reactivity and stable electron configurations.
- LanthanidesA series of elements from lanthanum, known for their rare earth properties.
- ActinidesA series of elements from actinium, often radioactive and synthetic.
- Electron arrangementsThe distribution of electrons in an atom, influencing chemical properties and group similarities.
- Inert gasesAnother term for noble gases, initially believed to be completely nonreactive.
- Chemical propertiesCharacteristics of elements that determine their behavior in chemical reactions.
- DynamicDescribes the periodic table's potential to change with new element discoveries.
- SeriesAnother term for groups or families in the periodic table, indicating vertical columns.