Periodic Table: Charges definitions Flashcards
 Back
BackPeriodic Table: Charges definitions
1/15
Terms in this set (15)
- Noble GasesElements in group 8A or 18 with a stable electron configuration and no charge.
- CationsPositively charged ions formed when metals lose electrons.
- AnionsNegatively charged ions formed when nonmetals gain electrons.
- Type 1 MetalsMetals with only one type of positive charge.
- Type 2 MetalsMetals, often transition metals, with multiple positive charges.
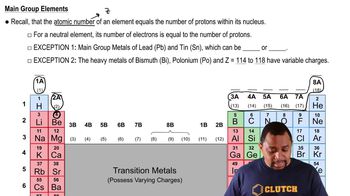
- Transition MetalsElements with variable positive charges due to electron arrangements.
- Main Group ElementsElements in groups 1A to 8A, excluding transition metals.
- Atomic NumberNumber of protons in an atom's nucleus, denoted by Z.
- MetalloidsElements with properties of both metals and nonmetals.
- Electron ConfigurationArrangement of electrons in an atom's electron shells.
- Group 1AElements that typically form +1 cations by losing one electron.
- Group 2AElements that typically form +2 cations by losing two electrons.
- Group 3AElements that typically form +3 cations by losing three electrons.
- Group 7AElements that typically form -1 anions by gaining one electron.
- Group 6AElements that typically form -2 anions by gaining two electrons.