Partial Pressure definitions Flashcards
 Back
BackPartial Pressure definitions
1/10
Terms in this set (10)
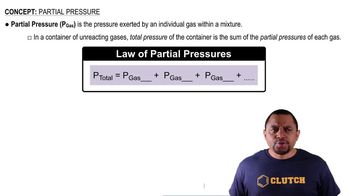
- Partial PressureThe pressure exerted by an individual gas within a mixture.
- Dalton's LawStates that the total pressure is the sum of the partial pressures of each gas in a mixture.
- Ideal Gas LawA formula used to calculate the behavior of an ideal gas using moles, gas constant, temperature, and volume.
- Mole FractionThe ratio of moles of a specific gas to the total moles of all gases in a mixture.
- Total PressureThe sum of all partial pressures of gases within a container.
- Gas ConstantA constant used in the ideal gas law, typically denoted as R.
- TemperatureA measure of the average kinetic energy of particles in a substance, affecting gas pressure.
- VolumeThe space occupied by a gas, influencing its pressure when combined with moles and temperature.
- MolesA measure of the amount of substance, used in calculating gas properties.
- Gas MixtureA combination of different gases within a single container.