Paramagnetism and Diamagnetism definitions Flashcards
 Back
BackParamagnetism and Diamagnetism definitions
1/10
Terms in this set (10)
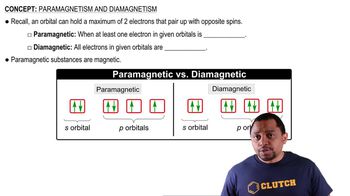
- Pauli Exclusion PrincipleA rule stating that an orbital can hold a maximum of two electrons with opposite spins.
- ParamagneticA property of substances with at least one unpaired electron, making them responsive to magnetic fields.
- DiamagneticA property of substances where all electrons are paired, rendering them unaffected by magnetic fields.
- OrbitalA region in an atom where there is a high probability of finding electrons.
- Electron ConfigurationThe arrangement of electrons in an atom's orbitals, crucial for determining magnetic properties.
- Magnetic FieldAn invisible field around magnetic materials and electric currents, influencing paramagnetic substances.
- Unpaired ElectronAn electron that occupies an orbital alone, contributing to paramagnetism.
- SpinA quantum property of electrons, with opposite spins allowing two electrons to occupy the same orbital.
- S OrbitalA spherical orbital that can hold up to two electrons with opposite spins.
- P OrbitalA dumbbell-shaped orbital that can hold up to six electrons, with potential for unpaired electrons.