Paramagnetism and Diamagnetism definitions Flashcards
 Back
BackParamagnetism and Diamagnetism definitions
1/10
Terms in this set (10)
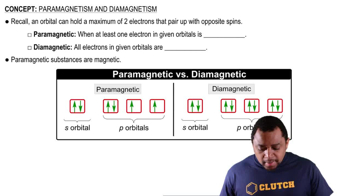
- ParamagnetismPhenomenon where substances with unpaired electrons are attracted to magnetic fields.
- DiamagnetismPhenomenon where substances with all paired electrons show no attraction or slight repulsion to magnetic fields.
- OrbitalRegion in an atom where there is a high probability of finding electrons.
- ElectronSubatomic particle with a negative charge, found in orbitals around an atom's nucleus.
- Pauli Exclusion PrincipleQuantum principle stating that no two electrons can have identical quantum numbers in an atom.
- Magnetic FieldInvisible field around magnetic materials or moving electric charges, influencing other materials.
- Unpaired ElectronElectron occupying an orbital alone, contributing to paramagnetic properties.
- Paired ElectronsTwo electrons occupying the same orbital with opposite spins, contributing to diamagnetic properties.
- S OrbitalSpherical atomic orbital that can hold up to two electrons with opposite spins.
- P OrbitalDumbbell-shaped atomic orbital that can hold up to six electrons across three orientations.