Oxide Reactions definitions Flashcards
 Back
BackOxide Reactions definitions
1/15
Terms in this set (15)
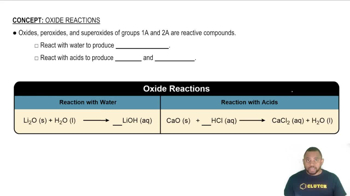
- OxidesCompounds that react with water to form hydroxides and with acids to produce salts and water.
- PeroxidesReactive compounds that can form hydroxides with water and salts with acids.
- SuperoxidesHighly reactive compounds that interact with water and acids to form bases and salts.
- Group 1AElements whose oxides react with water to form basic hydroxides and with acids to form salts.
- Group 2AElements whose oxides are reactive, forming bases with water and salts with acids.
- HydroxidesBasic compounds formed when oxides react with water.
- SaltsIonic compounds produced when oxides react with acids.
- Lithium OxideReacts with water to produce lithium hydroxide, a basic compound.
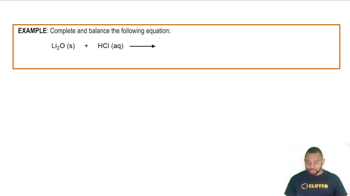
- Calcium OxideReacts with hydrochloric acid to form calcium chloride and water.
- Lithium HydroxideA basic compound formed from the reaction of lithium oxide with water.
- Calcium ChlorideA salt formed from the reaction of calcium oxide with hydrochloric acid.
- BasicDescribes the nature of hydroxides formed from oxide reactions with water.
- Reactive CompoundsSubstances like oxides that readily react with water and acids.
- Ionic CompoundA type of compound, such as a salt, formed from oxide reactions with acids.
- Hydrochloric AcidAn acid that reacts with calcium oxide to produce calcium chloride and water.