Osmotic Pressure definitions Flashcards
 Back
BackOsmotic Pressure definitions
1/11
Terms in this set (11)
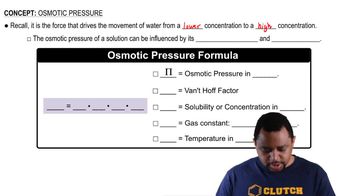
- Osmotic PressureThe force driving water movement from lower to higher solute concentration across a semipermeable membrane.
- Semipermeable MembraneA barrier that allows certain molecules or ions to pass through by diffusion.
- Van't Hoff FactorA measure of the effect of solute particles on the colligative properties of a solution.
- MolarityThe concentration of a solution expressed as moles of solute per liter of solution.
- Gas ConstantA constant used in equations of state, equal to 0.08206 L·atm/mol·K.
- TemperatureA measure of the thermal energy of a system, influencing osmotic pressure when expressed in Kelvin.
- KelvinThe SI base unit of temperature, used in scientific temperature measurements.
- ConcentrationThe abundance of a constituent divided by the total volume of a mixture.
- SoluteA substance dissolved in another substance, forming a solution.
- SolutionA homogeneous mixture composed of two or more substances.
- AtmospheresA unit of pressure defined as 101,325 Pa, used in measuring osmotic pressure.