Osmolarity definitions Flashcards
 Back
BackOsmolarity definitions
1/10
Terms in this set (10)
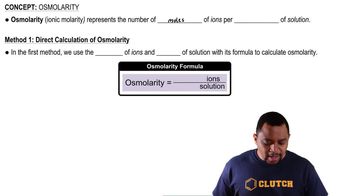
- OsmolarityMeasure of total concentration of solute particles in a solution, expressed as moles of ions per liter.
- MolarityConcentration of a solute in a solution, expressed as moles of solute per liter.
- IonsCharged particles formed when atoms gain or lose electrons.
- SolutionHomogeneous mixture composed of two or more substances.
- SoluteSubstance dissolved in a solvent to form a solution.
- Direct CalculationMethod using moles of ions and liters of solution to find osmolarity.
- CompoundSubstance formed from two or more elements chemically bonded together.
- OsmosisMovement of water across a semipermeable membrane from low to high solute concentration.
- BiochemistryStudy of chemical processes within and relating to living organisms.
- ChemistryScience of matter and its interactions with energy and itself.