Orientations of D Orbitals definitions Flashcards
 Back
BackOrientations of D Orbitals definitions
1/13
Terms in this set (13)
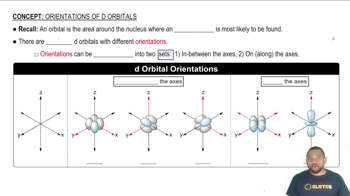
- d OrbitalsFive orbitals with unique spatial orientations around an atom's nucleus, crucial for understanding electron distribution.
- OrientationThe spatial arrangement of orbitals in relation to the x, y, and z axes.
- dxyd_{xy}An orbital oriented between the x and y axes, with lobes positioned diagonally.
- dyzd_{yz}An orbital oriented between the y and z axes, with lobes positioned diagonally.
- dxzd_{xz}An orbital oriented between the x and z axes, with lobes positioned diagonally.
- dx2−y2d_{x^2-y^2}An orbital with lobes lying directly on the x and y axes, intersected by these axes.
- dz2d_{z^2}An orbital aligned along the z-axis, with lobes piercing through the nucleus.
- AxesReference lines used to describe the orientation of orbitals, typically x, y, and z.
- LobesRegions of high electron probability within an orbital, defining its shape and orientation.
- NucleusThe central part of an atom where protons and neutrons are located, around which orbitals are arranged.
- Transition MetalsElements characterized by partially filled d orbitals, influencing their chemical properties.
- Molecular BondingThe interaction between atoms that leads to the formation of molecules, influenced by orbital orientation.
- Electronic StructureThe arrangement of electrons in an atom, crucial for understanding chemical behavior.