Nature of Energy definitions Flashcards
 Back
BackNature of Energy definitions
1/13
Terms in this set (13)
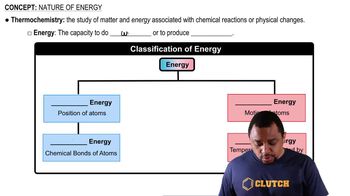
- ThermochemistryStudy of matter and energy in chemical reactions or physical changes.
- Potential EnergyEnergy related to the position of atoms.
- Kinetic EnergyEnergy associated with the motion of atoms.
- Chemical EnergyForm of potential energy from chemical bonds.
- Thermal EnergyType of kinetic energy linked to temperature changes.
- JouleStandard unit of energy, named after James Joule.
- CalorieUnit of energy; 1 calorie equals 4.184 joules.
- Food CalorieUnit of energy; 1 food Calorie equals 4184 joules.
- Kilowatt-hourUnit of energy; 1 kilowatt-hour equals 3.6 × 10^6 joules.
- EnergyCapacity to do work or produce heat.
- SI UnitInternational System of Units standard for measurements.
- Conversion FactorRatio used to convert one unit of measurement to another.
- James JouleEnglish scientist after whom the joule is named.