Naming Coordination Compounds definitions Flashcards
 Back
BackNaming Coordination Compounds definitions
1/15
Terms in this set (15)
- IUPACA systematic set of rules for naming chemical compounds, including coordination compounds.
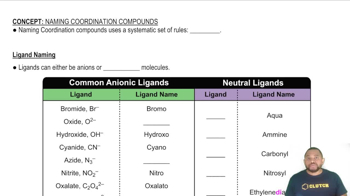
- LigandAn ion or molecule that binds to a central metal atom to form a coordination complex.
- AnionA negatively charged ion that can act as a ligand in coordination compounds.
- Neutral moleculeA molecule with no net charge that can act as a ligand in coordination compounds.
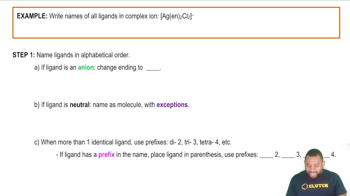
- Complex ionA charged species consisting of a central metal atom bonded to surrounding ligands.
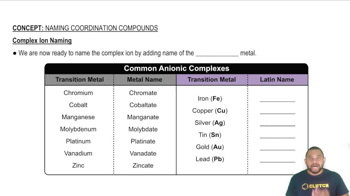
- Transition metalA metal element that can form complex ions with various ligands.
- Latin-based nameA name derived from Latin used for certain metal ions in coordination compounds.
- CationA positively charged ion that is named before the anion in coordination compounds.
- Counter ionAn ion that balances the charge of a complex ion in a coordination compound.
- Coordination complexA compound consisting of a complex ion and counter ions.
- BromoThe ligand name for the bromide ion in coordination compounds.
- AquaThe ligand name for water in coordination compounds.
- AmineThe ligand name for ammonia in coordination compounds.
- CarbonylThe ligand name for carbon monoxide in coordination compounds.
- EthylenediamineA bidentate ligand often abbreviated as 'en' in coordination compounds.