Naming Acids definitions Flashcards
 Back
BackNaming Acids definitions
1/15
Terms in this set (15)
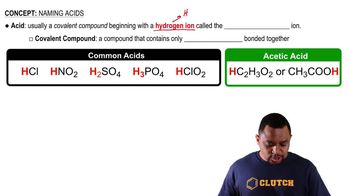
- AcidA covalent compound typically starting with a hydrogen ion, composed of nonmetals.
- Hydronium ionAnother name for the hydrogen ion, represented as H+.
- Covalent compoundA compound consisting only of nonmetals bonded together.
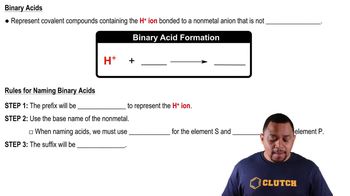
- Binary acidAn acid with hydrogen ions bonded to nonmetal anions, excluding oxygen.
- OxyacidA covalent compound with hydrogen ions bonded to a polyatomic ion containing oxygen.
- Polyatomic ionAn ion composed of multiple atoms, often containing oxygen.
- Hydro-Prefix used in naming binary acids, indicating the presence of hydrogen.
- NitrateA polyatomic ion with the formula NO3-, used in forming nitric acid.
- NitriteA polyatomic ion with the formula NO2-, used in forming nitrous acid.
- IodineA nonmetal element in group 7A, forming anions with a -1 charge.
- SulfurA nonmetal element used entirely in naming its acid form, not just the base name.
- PhosphorusA nonmetal element requiring more than its base name in acid form.
- Ic acidSuffix used for acids derived from polyatomic ions ending in '-ate'.
- Ous acidSuffix used for acids derived from polyatomic ions ending in '-ite'.
- Acetic acidAn exception to typical acid structure, can be written with hydrogen at the end.