Molecular Equations definitions Flashcards
 Back
BackMolecular Equations definitions
1/10
Terms in this set (10)
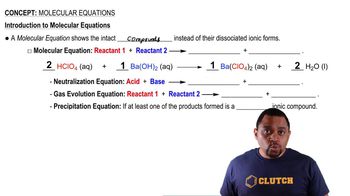
- Molecular EquationRepresents reactants and products as intact compounds, not dissociated ions, in a chemical reaction.
- Neutralization ReactionInvolves an acid and a base reacting to form an ionic compound and water.
- Gas Evolution ReactionA chemical reaction where one of the products formed is a gas.
- Precipitation ReactionA reaction resulting in the formation of a solid ionic compound, known as a precipitate.
- Aqueous ReactantA reactant dissolved in water, participating in a chemical reaction.
- Perchloric AcidA strong acid often used in molecular equations, represented in its aqueous form.
- Barium HydroxideA base that reacts with acids in neutralization reactions to form ionic compounds.
- Barium PerchlorateAn ionic compound formed from the reaction of perchloric acid and barium hydroxide.
- Solubility RulesGuidelines determining whether a compound will dissolve in water or form a precipitate.
- PrecipitateA solid ionic compound formed in a solution during a chemical reaction.