Molality definitions Flashcards
 Back
BackMolality definitions
1/10
Terms in this set (10)
- MolalityA concentration measure defined as moles of solute per kilogram of solvent, unaffected by temperature changes.
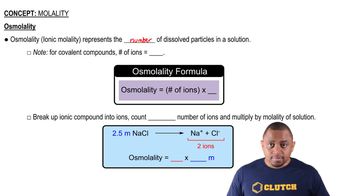
- OsmolalityAlso known as ionic molality, it accounts for the number of dissolved particles in a solution.
- SoluteThe substance dissolved in a solvent to form a solution.
- SolventThe substance in which a solute is dissolved to form a solution.
- Covalent CompoundsCompounds where atoms are bonded by shared electrons, typically not dissociating into ions.
- Ionic CompoundsCompounds that dissociate into ions when dissolved, affecting osmolality.
- Sodium ChlorideAn ionic compound that dissociates into sodium and chloride ions in solution.
- Temperature IndependenceA characteristic of molality, as it does not involve volume, remaining constant with temperature changes.
- MolesA unit representing the amount of substance, used in calculating molality.
- KilogramsThe unit of mass for the solvent in the calculation of molality.