Maxwell-Boltzmann Distribution definitions Flashcards
 Back
BackMaxwell-Boltzmann Distribution definitions
1/10
Terms in this set (10)
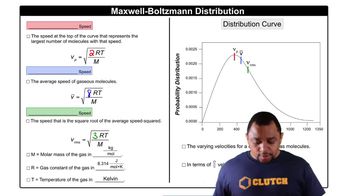
- Maxwell-Boltzmann DistributionA probability distribution describing the speed of ideal gas molecules at a given temperature.
- Probable SpeedThe most common velocity among gas molecules, represented at the peak of the distribution curve.
- Mean SpeedThe average velocity of gas molecules, calculated as the square root of 8RT/M.
- Root Mean Square SpeedThe square root of the average of the squares of velocities, calculated as the square root of 3RT/M.
- Distribution CurveA graph showing the probability of gas molecules at various velocities, with velocity on the X-axis.
- Gas ConstantA constant used in speed calculations, denoted as R, with a value of 8.314 J/(mol·K).
- Molar MassThe mass of a given substance divided by the amount of substance, measured in kg/mol.
- Probability DistributionA function that represents the likelihood of different outcomes, such as molecular speeds.
- VelocityThe speed of gas molecules, represented on the X-axis of the distribution curve.
- TemperatureA measure of thermal energy, affecting the speed distribution of gas molecules, measured in Kelvin.