Main Group Elements: Density definitions Flashcards
 Back
BackMain Group Elements: Density definitions
1/15
Terms in this set (15)
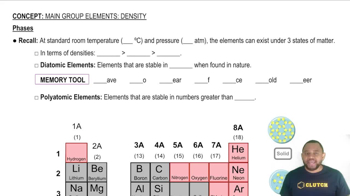
- Main Group ElementsElements in groups 1, 2, and 13-18 of the periodic table, excluding transition metals.
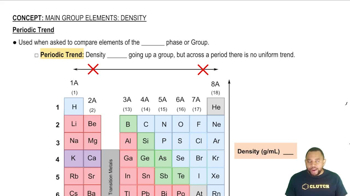
- DensityA measure of mass per unit volume, often used to compare the compactness of substances.
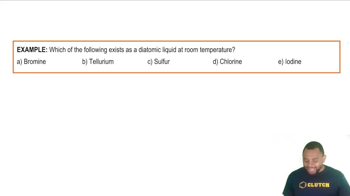
- Diatomic ElementsElements that naturally exist as molecules composed of two atoms, such as H2, N2, and O2.
- Polyatomic ElementsElements that naturally exist as molecules composed of more than two atoms, like P4 and S8.
- Hydrogen BondingA strong type of dipole-dipole interaction occurring between molecules containing hydrogen bonded to a highly electronegative atom.
- Periodic TrendPatterns observed in the periodic table, such as density trends within groups and periods.
- Standard PressureA pressure of 1 atmosphere, used as a reference point for measuring gas properties.
- Standard TemperatureA temperature of 25 degrees Celsius, used as a reference point for measuring substance properties.
- Monoatomic ElementsElements that exist as single atoms in their natural state, unlike diatomic or polyatomic elements.
- VolumeThe amount of space occupied by a substance, influencing its density when combined with mass.
- MassThe quantity of matter in a substance, used in calculating density with volume.
- Phases of MatterThe distinct forms that different states of matter take on, namely solids, liquids, and gases.
- ExceptionsInstances where general rules or trends do not apply, such as water's density anomaly.
- HydrogenThe lightest element, often found as a diatomic gas (H2) under standard conditions.
- BromineA main group element that is a liquid at room temperature and standard pressure.