Magnetic Properties of Complex Ions definitions Flashcards
 Back
BackMagnetic Properties of Complex Ions definitions
1/12
Terms in this set (12)
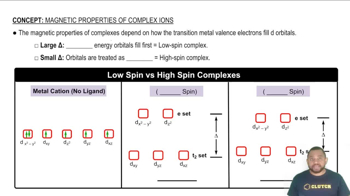
- Crystal Field Splitting EnergyThe energy difference between two sets of d orbitals in a complex ion, influencing electron arrangement.
- Low-Spin ComplexA complex where electrons fill lower energy orbitals first, resulting in paired electrons and diamagnetic properties.
- High-Spin ComplexA complex where electrons occupy higher energy orbitals, leading to unpaired electrons and paramagnetic properties.
- DiamagneticA property of a substance with all electrons paired, resulting in no net magnetic moment.
- ParamagneticA property of a substance with unpaired electrons, resulting in a net magnetic moment.
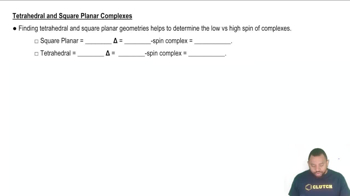
- Square Planar GeometryA molecular shape associated with large crystal field splitting energy, leading to low-spin, diamagnetic complexes.
- Tetrahedral GeometryA molecular shape associated with small crystal field splitting energy, leading to high-spin, paramagnetic complexes.
- Degenerate OrbitalsOrbitals that have the same energy level, often seen in high-spin complexes with small splitting energy.
- d OrbitalsThe five orbitals in transition metals that are split into different energy levels in complex ions.
- Hund's RuleA principle stating that electrons fill degenerate orbitals singly before pairing up.
- Metal CationA positively charged metal ion that forms the central part of a complex ion.
- LigandsMolecules or ions that donate electron pairs to a metal cation in a complex ion.