Limiting Reagent definitions Flashcards
 Back
BackLimiting Reagent definitions
1/13
Terms in this set (13)
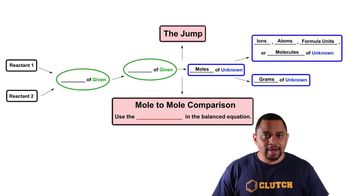
- Limiting ReagentThe reactant that is completely consumed in a reaction, determining the maximum amount of product formed.
- Theoretical YieldThe maximum amount of product that can be formed from a chemical reaction, assuming complete consumption of the limiting reagent.
- Excess ReagentThe reactant that remains after the completion of the chemical reaction, not fully consumed.
- StoichiometryThe calculation of reactants and products in chemical reactions using balanced equations.
- Chemical EquationA representation of a chemical reaction showing the reactants and products with their respective amounts.
- MolesA unit of measurement for amount of substance, used in stoichiometry to relate quantities of reactants and products.
- GramsA unit of mass used to measure the amount of a substance in stoichiometric calculations.
- CoefficientsNumbers in a balanced chemical equation that indicate the ratio of moles of reactants and products.
- IonsCharged particles formed when atoms gain or lose electrons, often involved in chemical reactions.
- AtomsThe basic units of chemical elements, involved in forming molecules and compounds in reactions.
- Formula UnitsThe lowest whole number ratio of ions in an ionic compound, used in stoichiometric calculations.
- MoleculesGroups of atoms bonded together, representing the smallest fundamental unit of a chemical compound.
- Balance EquationAn equation where the number of atoms for each element is equal on both sides, ensuring mass conservation.