Ligands definitions Flashcards
 Back
BackLigands definitions
1/15
Terms in this set (15)
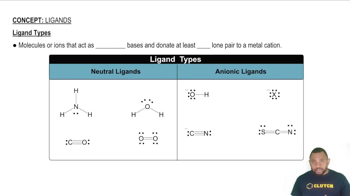
- LigandsMolecules or ions acting as Lewis bases, donating lone pairs to metal cations.
- Lewis BaseAn electron pair donor in a chemical reaction.
- Metal CationA positively charged metal ion that acts as a Lewis acid.
- Lewis AcidAn electron pair acceptor in a chemical reaction.
- Neutral LigandsLigands with no charge, such as ammonia, water, and carbon monoxide.
- Anionic LigandsNegatively charged ligands like hydroxide ion, halogens, and cyanide ion.
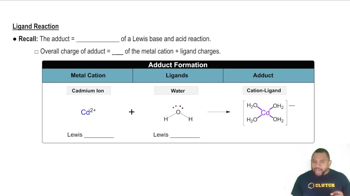
- AdductThe product formed when ligands bind to a metal cation.
- AmmoniaA neutral ligand with a lone pair that can be donated to a metal cation.
- Carbon MonoxideA neutral ligand where carbon donates a lone pair to a metal cation.
- Hydroxide IonAn anionic ligand with a negative charge, donating a lone pair.
- Cyanide IonAn anionic ligand with a negative charge on carbon, donating a lone pair.
- Thiocyanate IonAn anionic ligand with a negative charge, donating lone pairs to metal cations.
- Cadmium IonA metal cation that can form adducts with ligands like water.
- HalogensGroup 7A elements that can act as anionic ligands.
- OxygenA neutral ligand where either atom can donate a lone pair to a metal cation.